Why Is It Important To Balance A Chemical Equation
Camila Farah
However the primary reason for balancing is to ensure the law of conservation of mass.
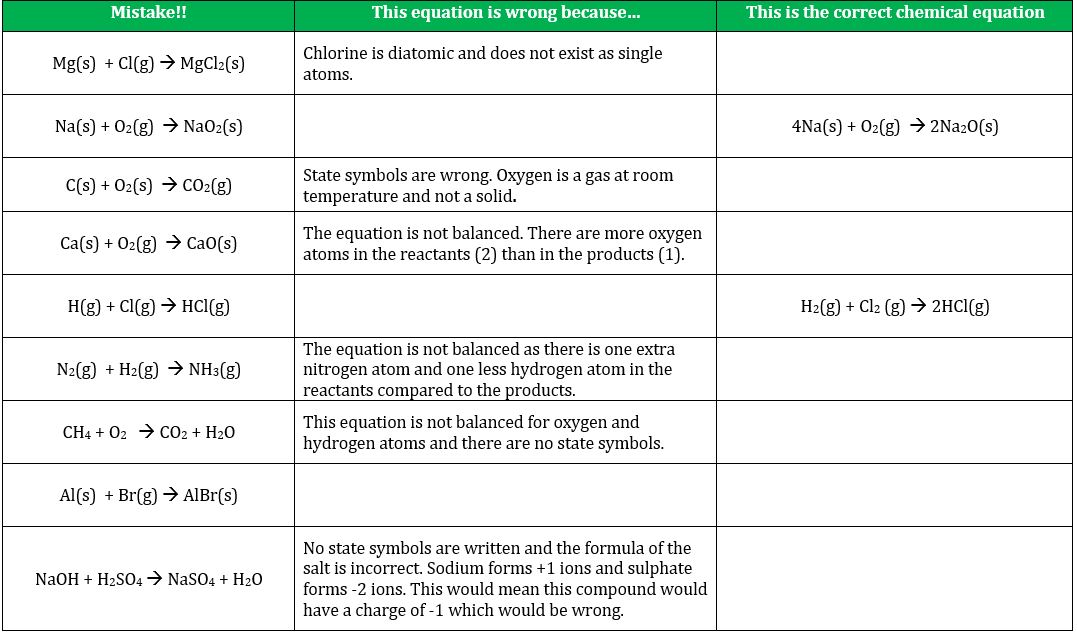
This means that the. In other words the mass and the charge are balanced on both sides of the reaction. It is not important to balance a chemical equation. There are endless reasons as to why you need to balance your chemical equations.
The quality and quantity of the elements remain precisely the same. It is important to balance chemical equations because there must be an equal number of atoms on both sides of the equation to follow the law of the conservation of mass. To make sure you have the correct reactants. But the amount of reactant matter equals to that of the products.
In the physical laws law of conservation of matter states that the initial matter will get converted to the products or gets rearranged into some other particles of different kind. 1 to keep your chemistry teacher happy 2 because a balanced equation gives you the molar ratios of all the reactants and products for the reaction. This chemical law states that in order for the equation to be correct an equal quantity of matter exists both before and after the experiment. To make sure the correct products.
RELATED ARTICLE :
- why are ships painted red below the waterline
- why are the corners of my mouth red
- why are some pots and pans designed with wooden handles
The reason behind the balancing of a chemical equation is according to law of conservation of matter.
Source : pinterest.com